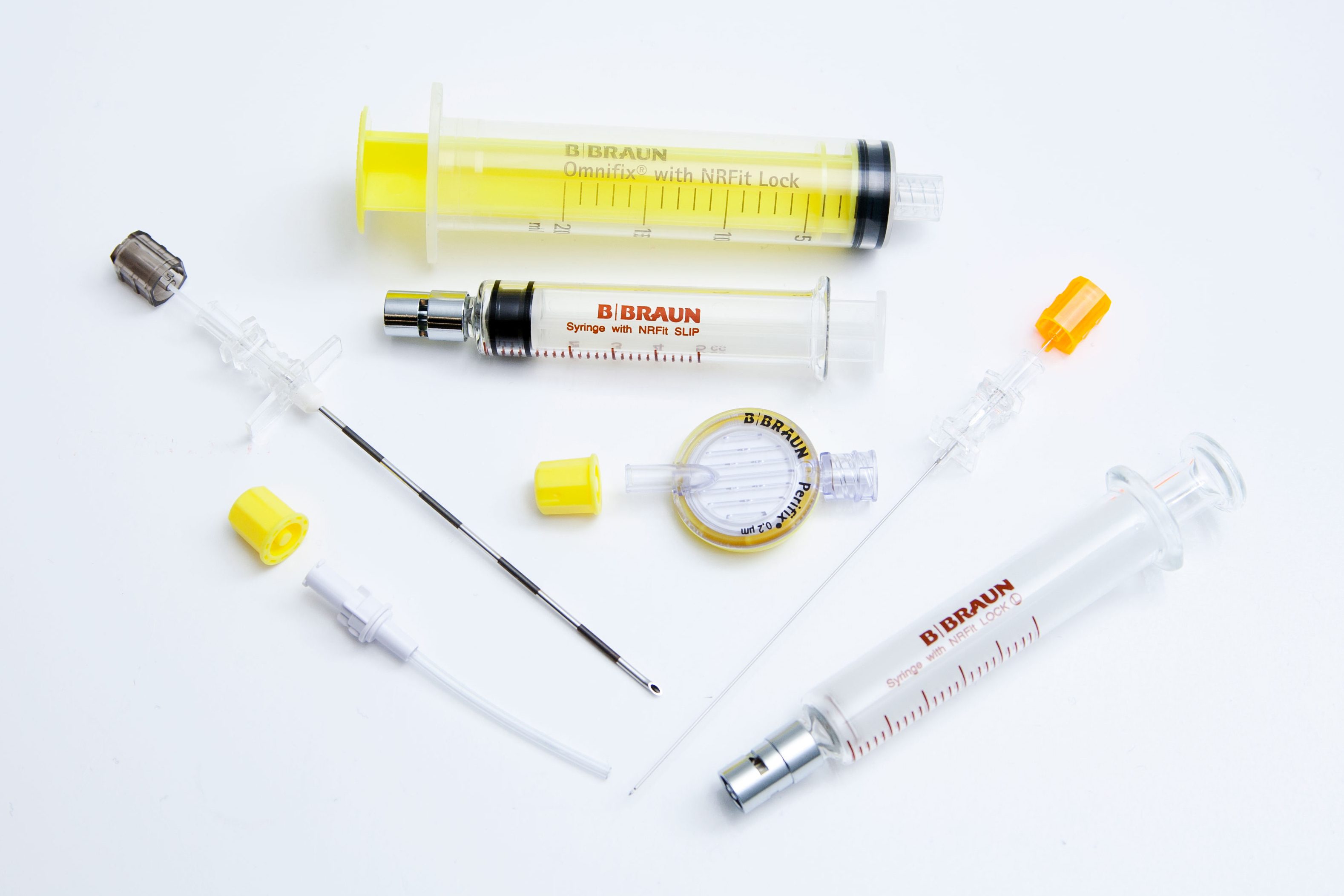
NRFit™ Connectors
GEDSA and the StayConnected Initiative
StayConnected is the implementation branch of the Global Enteral Device Supplier Association (GEDSA) which is a federally tax exempt non-profit trade association. StayConnected was established in part to help introduce the new ISO standard connectors and facilitate adoption of the new 80369-6 connectors with the healthcare community. GEDSA, which is composed of leading manufacturers and distributors of medical devices, is united by a shared desire to increase patient safety and optimal delivery of neuraxial treatments and procedures. GEDSA speaks with a unified voice to communicate with government agencies, professional associations, healthcare institutions and member suppliers regarding issues that face device manufacturers, suppliers and distributors.
GEDSA leads StayConnected, a joint communication initiative on behalf of the industry to ensure consistency and avoid any confusion as new, safer connectors are introduced in the market.
Through GEDSA, and where possible, the industry has agreed to:
- Coordinate market communications
- Use a common brand name for the ISO 80369-6 connector (NRFit™)
- Introduce devices with NRFit connectors to the market around the same time
- Where possible, use yellow device coloring to help identify NRFit components in a kit
Visit http://www.stayconnected.org to learn more.
International Organization for Standardization (ISO) standard 80369-6
The goal of this standard is to reduce the risk of misconnections and increase patient safety by helping to make neuraxial and regional anesthesia products incompatible with medical devices meant for other applications (i.e., IV).
Commonly referred to as products with NRFit connectors, the small bore connector on these devices may look similar to a Luer connector, but are about 20% smaller in diameter.
California State Legislation (AB 444)
As of Jan.1, 2017, this law prohibits healthcare facilities from using an epidural connector that would fit into a connector other than the type it was intended for, unless it’s an emergency. California health facilities were required to switch to epidural products with NRFit connectors by Jan. 1, 2017.
Types of Products Affected by the ISO 80369-6 Standard
Devices used to administer medications and to take samples from neuraxial sites (central nervous system including intracranial and spinal canal such as epidural and intrathecal), major peripheral nerve local anesthetic blockade and continuous wound infiltration. This includes anesthetic delivery, monitoring cerebrospinal fluid (CSF) pressure, and removing CSF for therapeutic or diagnostic purposes.
Products Affected by the California State Legislation AB 444
The California law specifically relates to epidurals. However, B. Braun has launched spinal, epidural and CSE products with NRFit to ease the transition for health systems.
Products NOT included in the ISO 80369-6 Standard
Some devices aren’t covered in the scope of the new standard because they’re not intended to inject into the epidural or spinal spaces. The most relevant of these are products used for the skin wheal application. These will remain Luer.
Examples may include:
- Spinal introducer needle
- Hypodermic needles
- Filter straw meant to draw up skin wheal medication
- 3 mL syringe used for skin wheal
Neuraxial Applications that will Remain Luer in the Short Term Until an NRFit™ Version is Available
All medical devices that connect to the neuraxial route will eventually need to convert to products with NRFit connectors. A small number of low-risk specialty devices/applications may continue to use Luer in the short term.
Penalties for California Customers who Don’t Comply
Unfortunately, there are no clear answers regarding penalties or enforcement of the law. However, there could be increased liability risk for hospitals to continue to use Luer products when NRFit is available on the market.
B. Braun Product Availability
Epidural, spinal and combined spinal epidural products with NRFit connectors were made available in October 2016. Additional neuraxial products as well as peripheral nerve block products will be available from B. Braun throughout 2020.
Other Vendor Product Availability
Although GEDSA members agreed to have products available around the same time, it’s important that the customers reach out to each of their suppliers to gather information regarding specific product availability.
Adapters (Luer to NRFit) According to the ISO Standard
These adapters are not allowed because it would be counter-productive to the safety initiative.
Discontinuation of the Current Luer Products
Over the next several years, B. Braun will continue to manufacture both Luer and NRFit products.
Managing the Transition
B. Braun sales representatives will work closely with customers to identify the best NRFit cross and make customers aware of any changes from existing product. We will work to ease the transition and develop a conversion schedule that will be agreed upon by the customer and sales representative.
NRFit Color Association
Although color coding is not included in the ISO standard, many manufacturers have agreed to use yellow product coloring where possible to help identify NRFit products.
B. Braun products incorporating yellow:
- 20 mL procedural syringe
- Epidural filter
- Catheter connector (cap)
- Filter straws & needles (cap)
- Polycarbonate spinal syringe
Does NOT include yellow:
- Plastic LOR syringes – package labeling and product labeling will indicate NRFit
- Glass syringes – package labeling and product labeling will indicate NRFit
- Spinal and epidural needles – package labeling will indicate NRFit
NRFit™ vs. Other Luer Connectors
The B. Braun Difference
Color Coordination – yellow product coloring where possible
Product Labeling – syringes are labeled as NRFit along with Slip or Lock on the barrel
Caps – yellow caps are used where product coloring isn’t feasible
Translucent Stylet Hubs – vs. opaque coloring
Products for Skin Wheal (Luer) – Luer products for skin wheal application are contained in a separate baggie within the kit
Package Labeling – products are labeled "NRFit" and have product codes ending in “N”