B. Braun Medical Inc. Issues Voluntary Nationwide Recall of 0.9% Sodium Chloride for Injection USP 250ML in Excel due to Fluid Leakage or Low Fill Volume
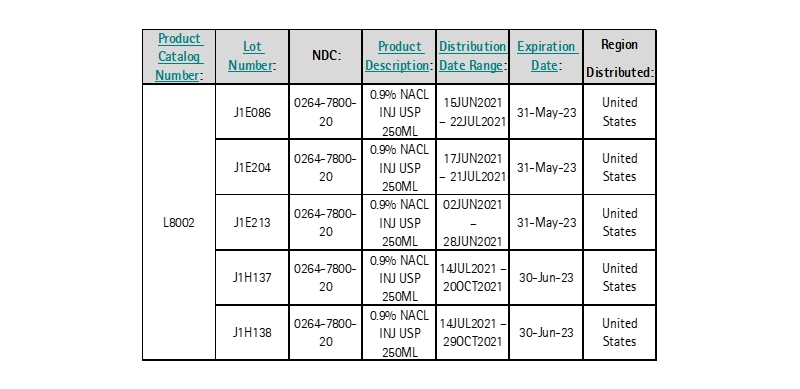
FOR IMMEDIATE RELEASE - March 2, 2022 - BETHLEHEM, PA – B. Braun Medical Inc. (B. Braun) is voluntarily recalling five (5) lots of 0.9% Sodium Chloride for Injection USP 250ML in Excel within the United States to the hospital/user level. The voluntary recall has been initiated due to fluid leakage or low fill volume of the respective containers.
The biggest risk with a slow leak in any intravenous solution preparation is a break in sterility which poses a risk for the patient being exposed to a bacterial or fungal infection. There is a remote probability this could lead to bloodstream infection. B. Braun has not received any reports of adverse events related to this recall.
0.9% Sodium Chloride for Injection USP in Excel is indicated for extracellular fluid replacement, treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion. 0.9% Sodium Chloride Injection USP in Excel is also indicated for use as a priming solution in hemodialysis procedures and may be used to initiate and terminate blood transfusions without hemolyzing red blood cells.
Product was distributed Nationwide within the United States to domestic distributors.
B. Braun is notifying its distributors and customers by an official recall notice sent via certified registered mail and is arranging for return of all recalled products. Facilities and distributors that have product which is being recalled should discontinue use immediately and contact the B. Braun Medical Inc. Customer Support Department at 800-227-2862 Monday through Friday, 8 a.m. – 6 p.m. EST to arrange for product return.
Facilities with questions regarding this recall can contact B. Braun by phone at 800-227-2862 Monday through Friday, 8 a.m. – 6 p.m. EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.