You have successfully logged out.
Products not made with Diethylhexyl Phthalate (DEHP) and Polyvinyl Chloride (PVC)
We're committed to the use of safe materials
As a healthcare provider, you want the best for your patients, from the treatments you select to the products you use. However, in everyday medical settings, products such as IV bags and tubing can contain up to 40% DEHP by weight, resulting in significant levels of exposure for neonates and other vulnerable patient populations.6
A 40-year history of doing the right thing
More than 40 years ago, B. Braun recognized the environmental and patient risks posed by medical products containing DEHP and PVC. We were the first medical device manufacturer to remove these harmful substances from many of our products, and remain the only supplier offering a full line of IV drug, solution and irrigation containers not made from DEHP or PVC. We extended our commitment to develop safer products in 2021 with the launch of CARESAFE™ IV Administration Sets, the first robust portfolio of IV administration sets in the U.S. not made with DEHP or PVC.
Reducing patient exposure to toxic chemicals
A growing body of evidence shows that Americans receiving care in hospitals and other settings can be exposed to dangerous levels of phthalates, a family of toxic chemicals. DEHP, which is used to soften PVC, is the phthalate most commonly used in medical products.2 In fact, based on an analysis of industry data, approximately 70-75% of IV and irrigation containers containing base solutions supplied in the U.S. are made using DEHP and PVC.3
The American Medical Association, among other professional organizations, encourages hospitals and physicians to reduce and phase out the use of PVC medical device products, especially those containing DEHP.4
Facts about DEHP and PVC
Fact 1: PVC has been determined to be a human carcinogen by the National Cancer Institute, Agency for Toxic Substances, Environmental Protection Agency, Disease Registry and the International Agency for Research on Cancer.5,6,7
Fact 2: The evidence of patient exposure to DEHP and other toxins during the course of clinical care is well established, and there is a continued need to reduce patient exposure.8
Fact 3: The Consumer Product Safety Commission has restricted eight phthalates, including DEHP, from children's toys because ingestion can cause harmful health effects.9
Fact 4: The State of California has determined that DEHP is a reproductive and developmental toxicant and a carcinogen and advises patients to request devices that do not contain DEHP when they are undergoing medical treatment.10
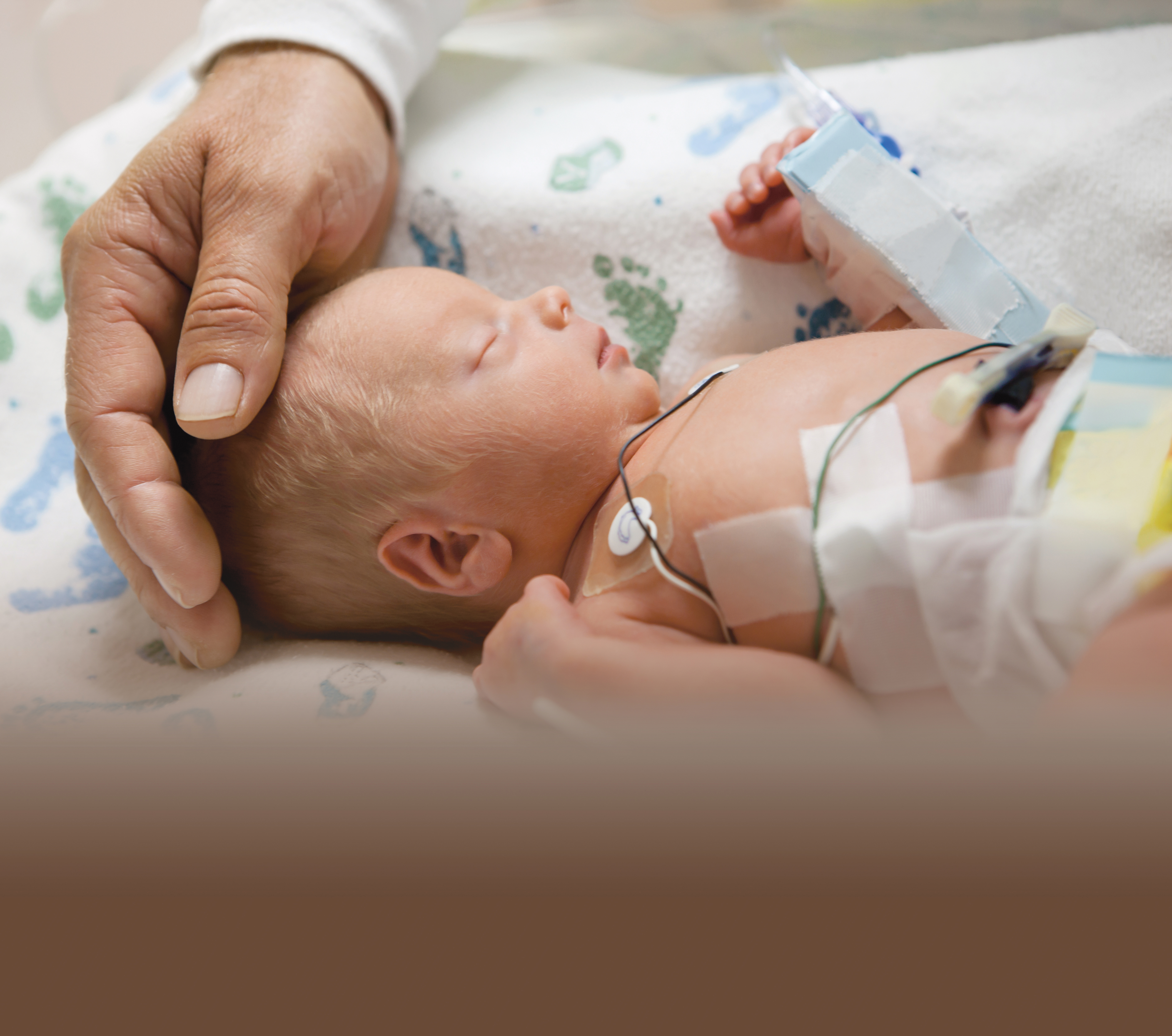
Who is at risk?
Products not made with DEHP or PVC help protect susceptible populations, such as: neonates and pediatric patients, particularly males, pregnant and lactating women and patients undergoing chemotherapy.
- In a NICU setting, a neonate is exposed to DEHP from multiple medical procedures. These procedures can result in exposure to significant levels of DEHP.11
- Neonatal exposure to DEHP is from both direct and indirect contact with PVC devices.12
- Based on the dose of DEHP received in such procedures as intravenous administration of sedatives, administration of TPN and replacement transfusions, all common procedures in the NICU, it is possible to estimate that a 4 kg infant could receive a DEHP dose on the order of 3 mg/kg/day for a period of weeks or months... 5-fold greater than the TI (Tolerable Intake)/dose ratio in this setting of 0.2.11
- Pregnant women exposed to DEHP may expose the fetus, potentially causing serious DEHP effects.13
- In the U.S., DEHP is no longer used in children's toys and child care articles such as baby bottle nipples.14
- Recent investigations have presented proof that DEHP can inhibit testosterone production in the adult human testis.15
- Phthalates have been associated with adverse reproductive system outcomes, including reduced semen quality and altered genital development.16
- Phthalates are endocrine-disrupting compounds, chemicals that animal studies have shown may alter hormonal signaling with potential effects on developing reproductive and nervous systems, metabolism and cancer.17
- Several medications, including Taxol®, can cause leaching of DEHP from PVC containers.18
- Patients can be affected adversely by adsorption of medications onto the walls of PVC IV containers. This adsorption can result in delivery of less than the prescribed doses of necessary medications.11
Taking action to protect patients
B. Braun is working with government officials, healthcare providers, educators, scientists, industry leaders and other stakeholders to support the adoption of public policies that ensure the safety of patients is protected each time they engage with the healthcare system.
We have called on the FDA to review and update its guidelines on the use of phthalates and other harmful chemicals in medical products and called for the establishment of a multi-agency task force to examine and recommend steps to reduce patient exposure to toxic materials in the healthcare system.
References
- Karpf, A. MD. Making patient safety a policy priority. Modern Healthcare. February 2021.
- Schettler, T. Polyvinyl chloride in health care. Health Care Without Harm. January 2020.
- B. Braun Medical Inc., analysis of industry data provided by IQVIA, Q1 2024 (24-0399).
- American Medical Association. (2016). Encouraging Alternatives to PVC/DEHP Products in Health H-135.945.
- Vinyl chloride: what is vinyl chloride? National Cancer Institute. Reviewed December 28, 2018. Accessed November 12, 2021. https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/vinyl-chloride
- Public health statement: Vinyl Chloride. Agency for Toxic Substances and Disease Registry. July 2006. Accessed November 12, 2021. https://www.atsdr.cdc.gov/toxprofiles/tp20-c1-b.pdf
- Polyvinyl chloride and copolymers production: national emission standards for hazardous air pollutants (NESHAP) – 40 CFR 63 subparts J & H. U.S Environmental Protection Agency. Updated February 23, 2021. Accessed November 12, 2021. https://www.epa.gov/stationary-sources-air-pollution/polyvinyl-chloride-and-copolymers-production-national-emission-0.
- NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Di(2-Ethylhexyl) Phthalate (DEHP), National Toxicology Program, Department of Health and Human Services, Retrieved at: https://ntp.niehs.nih.gov/ntp/ohat/phthalates/dehp/dehp-monograph.pdf
- Bornehag et al. 2004; Engel et al. 2010; Hauser and Calafat 2005; Hauser et al. 2006; Kimber and Dearmna 2010; Meeker et al. 2009a, 2009b; Mendiola et al. 2011; Swan 2008; Swan et al. 2005
- OEHHA Science for Health California. Proposition 65 Warnings Office of Environmental Health Hazard Assessment: Di(2-ethylhexyl)phthalate (DEHP). June, 2017. https://www.p65warnings.ca.gov/fact-sheets/di2-ethylhexylphthalate-dehp
- FDA CEDRH. Safety Assessment of Di(2-ethylhexly) Phthalate (DEHP) Released from PVC Medical Devices. FDA CEDRH 1 – 118, 2003.
- B Mallow and MA Fox. (2014). Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks. Journal of Perinatology, 1-6.
- Sharpe RM, Skakkebaek NE (2008) Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril 2008; 89(2 Suppl):e33-e38.
- US Consumer Product Safety Commission (CPSC Prohibition of Children’s Toys and Child Care Articles Containing Specific Phthalates. October 27, 2017. Accessed December 17, 2021.
- Desdoits-Lethimonier C, Albert O, Le Bizec B, Perdu E, Zalko D, Courant F, Lesné L, Guillé F, Dejucq-Rainsford N, Jégou B (3/2012) Human testis steroidogenesis is inhibited by phthalates. Hum Reprod; 2012 Mar 8. [Epub ahead of print]
- Bornehag et al. 2004; Engel et al. 2010; Hauser and Calafat 2005; Hauser et al. 2006; Kimber and Dearmna 2010; Meeker et al. 2009a, 2009b; Mendiola et al. 2011; Swan 2008; Swan et al. 2005
- Hannas BR, Furr J, Lambright CS, Wilson VS, Foster PM, Gray LE (2011) Dipentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the male Sprague-Dawley rat with greater relative potency than other phthalates. Toxicol Sci 120(1):184-193.
- Maas, B., Huber, C. & Krämer, I. Plasticizer extraction of Taxol®-infusion solution from various infusion devices. Pharm World Sci 18, 78–82 (1996). https://doi.org/10.1007/BF00579710

