You have successfully logged out.
Pharma & MedTech Partner
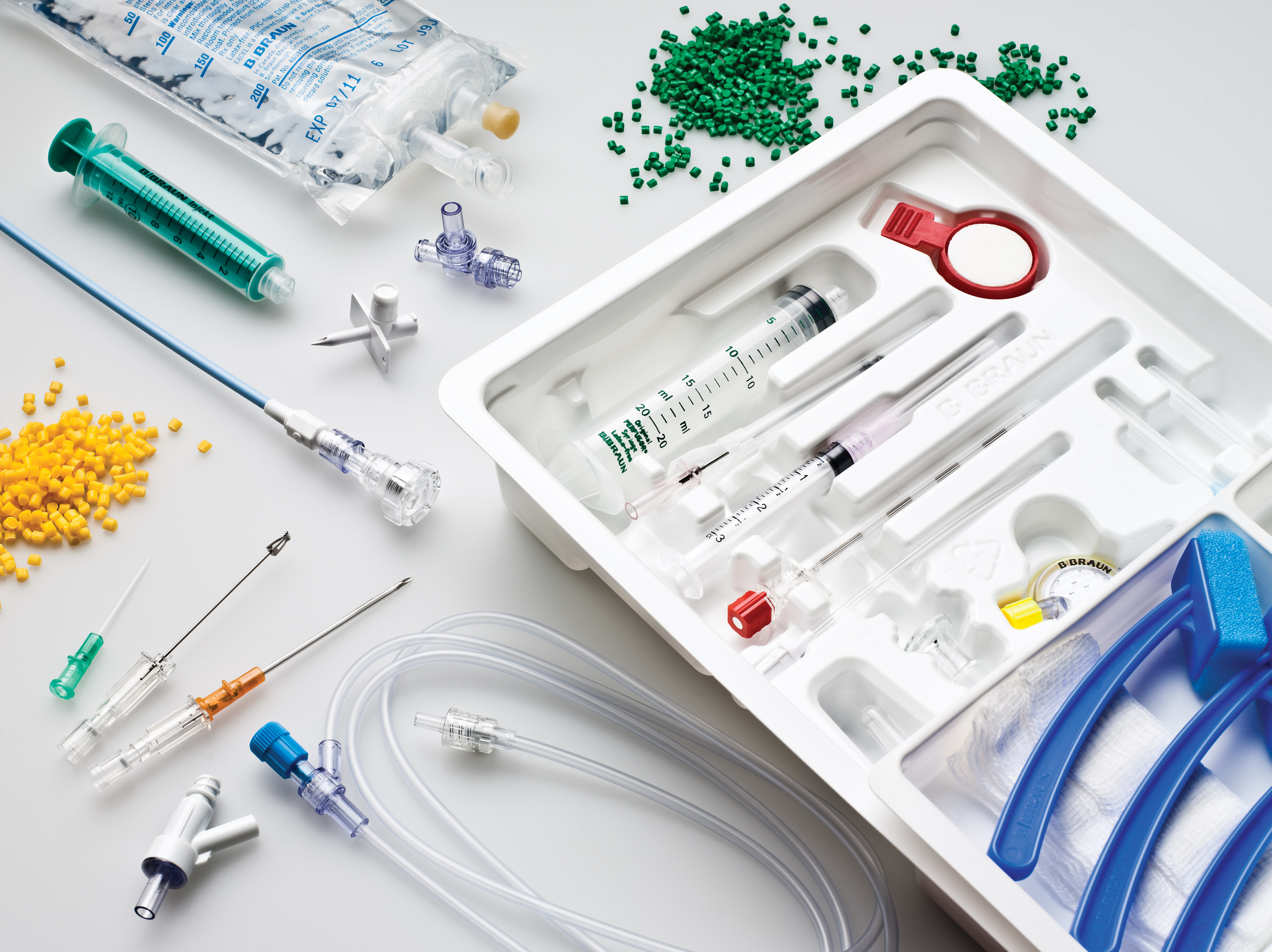
Your single project source from start to finish
Health industry partnership in medical and pharmaceutical supplies
Whether you are looking for a fluid administration set, custom kit or individual components, we provide contract manufacturing services to turn your ideas into reality. Our extensive product portfolio is backed by B. Braun’s global development, manufacturing and regulatory affairs networks. Our experts will partner with you to customize a solution to meet the unique needs of your business.
-
Located in
0
countries worldwide
-
Offering
0+
products globally
-
Manufactured
> 0%
in-house
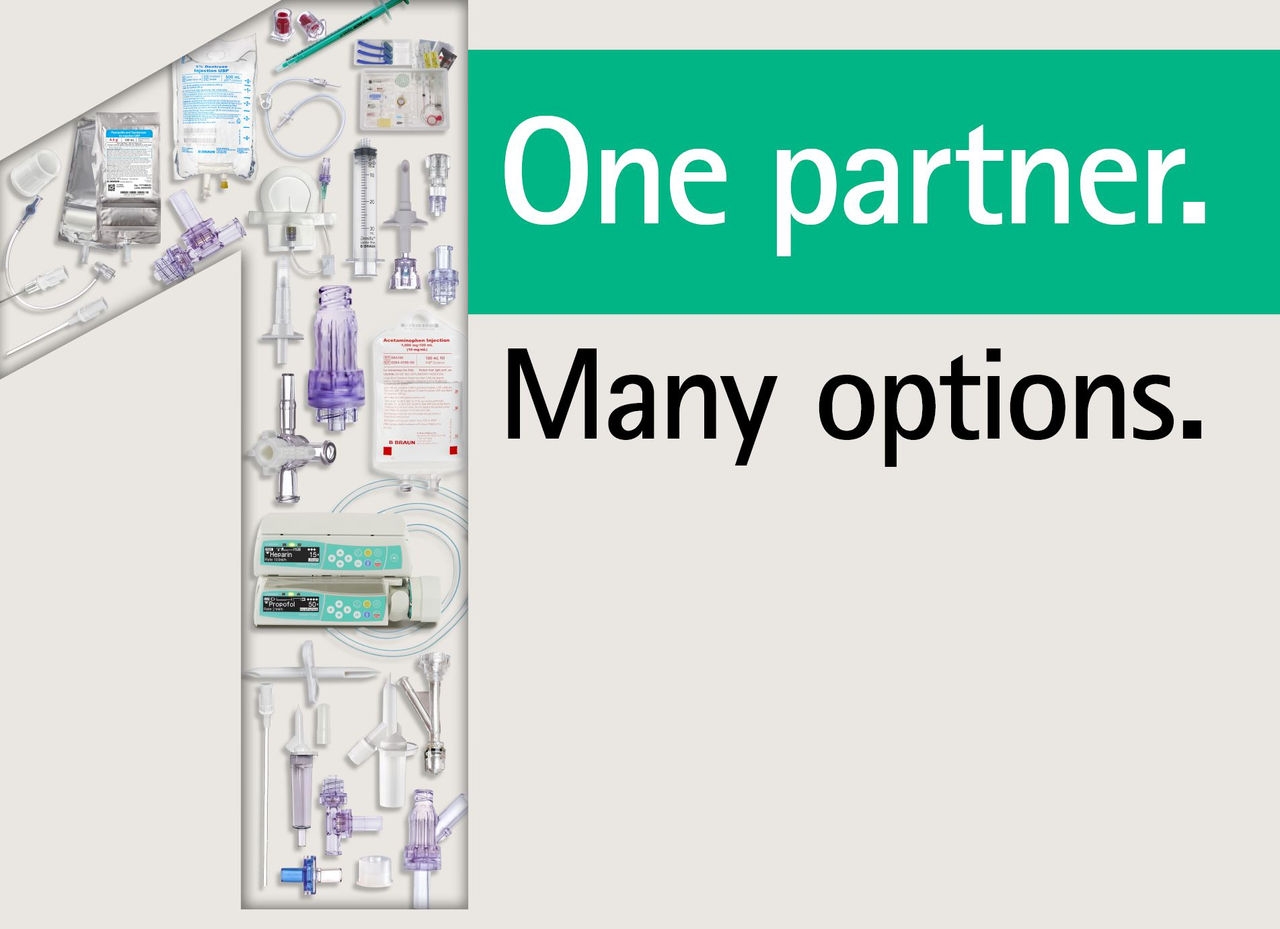
One partner. Many options.
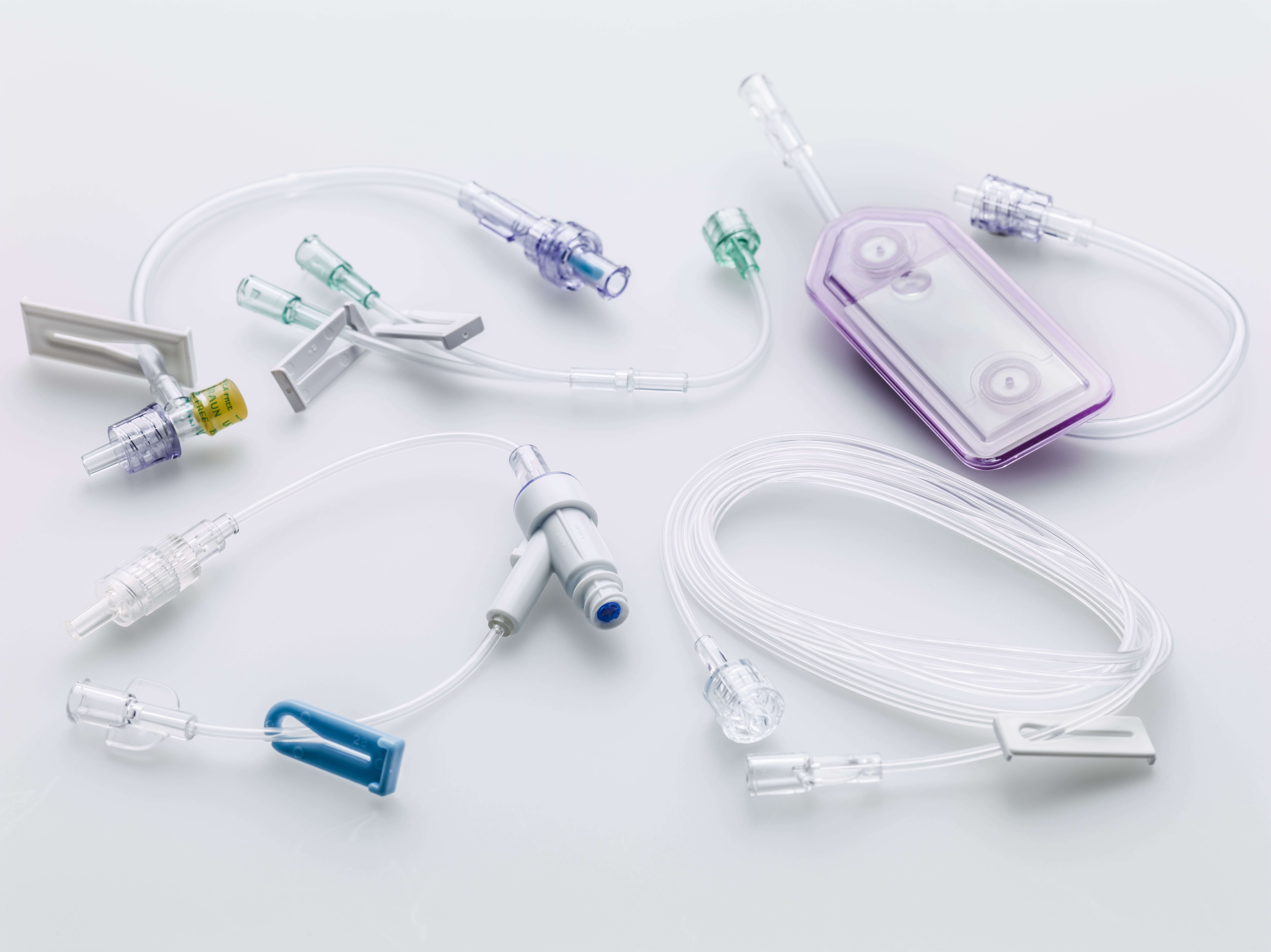
For us, partnership means more than taking responsibility for production. Our global expert network and in-house capabilities encompass a wide range of services, including design, assembly, regulatory, global product launch support, packaging and sterilization.

Comprehensive services and capabilities
B. Braun's Pharma & MedTech Partner Division offers a variety of in-house molding capabilities including injection molding, insert molding and over molding. Our primary 400,000-square-foot U.S. plant includes a 16,500-square-foot ISO Class 8 molding facility housing some of B. Braun’s 80 injection molding presses, which range from 55-330 tons.

As a leading provider of IV therapy products, B. Braun has extensive in-house extrusion capabilities. We can produce a wide range of extrusion types in varying sizes, materials and colors.

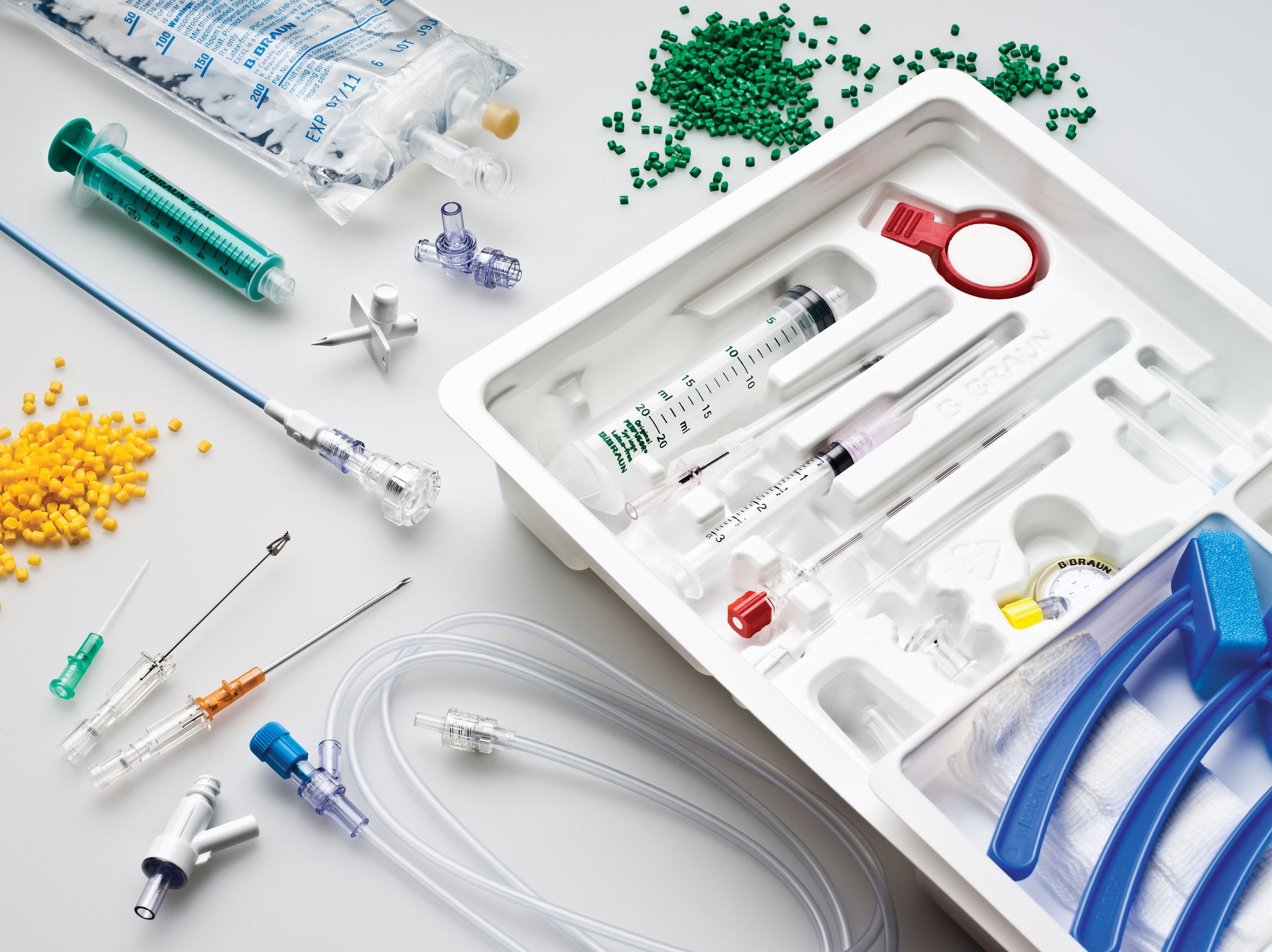
B. Braun exclusively designs and manufactures products for the medical market. This single-minded focus on medical devices established our reputation for unrivaled quality. We’ve built advanced manufacturing facilities around the world, and our primary North American facility is no exception. It features state-of-the-art quality systems and ISO Class 8 clean rooms. With the flexibility to change from manual to semi-automated to fully-automated assembly, we can produce both small- and large-scale volumes.

Our extensive packaging capabilities allow you to rely on B. Braun's Pharma & MedTech Partner Division as a single source for custom private-labeled devices, procedure kits and solutions.
B. Braun’s on-site quality control analytical lab features advanced testing equipment and provides comprehensive capabilities that support the Quality Assurance Program.
With our on-site laboratory services, your project receives the highest level of attention – helping to ensure product safety and improve speed to market

B. Braun's Pharma & MedTech Partner Division offers in-house EtO sterilization as a part of our single-source solution for customers. Our sterilization facility features eight 130- to 3,600-cubic-foot, fully validated chambers — all microprocessor controlled for precise operation. An on-site laboratory verifies sterility of every cycle according to ISO, FDA/QSR and USP requirements.
We also offer expertise in designing products with other sterilization methods in mind.

Your project includes a dedicated team – an account manager, product engineer, quality engineer, regulatory affairs specialist, customer service representative and sales coordinator – who helps meet your design, quality and regulatory requirements and keeps your project on-time and within budget.

B. Braun's Pharma & MedTech Partner Division has engineering centers worldwide to help design medical components, devices and kits. Our staff of knowledgeable design and manufacturing engineers and technicians works together closely to design a product that meets your requirements and keeps manufacturability in mind.

B. Braun has a complete in-house regulatory staff for both devices and drugs. Our regulatory affairs team will review all labeling to ensure compliance with FDA and global regulations. We offer support in filing drug applications, 510(k)s, PMAs and CE markings and assist in the regulatory assessment of product changes. B. Braun can also provide NAFTA certificates and country-of-origin letters.

B. Braun helps eliminate supply chain inefficiencies. Using our extensive base of approved suppliers, we can generate purchasing efficiencies. B. Braun can save your company time and resources through collaborative inventory management for pull-based minimum/maximum and kanban management.

Around the world, the word "quality" and "B. Braun" are synonymous. That holds true at B. Braun Pharma & MedTech Partner where we have established stringent processes and monitoring practices for consistent quality. To meet international standards B. Braun has ISO 13485 certified facilities and systems. In addition, each location adheres to country-specific regulatory and licensing mandates.

Helping you increase safety at the point of care
We’re committed to enhancing safety for patients, staff and facilities by designing products that reduce treatment errors and increase patient and healthcare worker safety.
Learn more
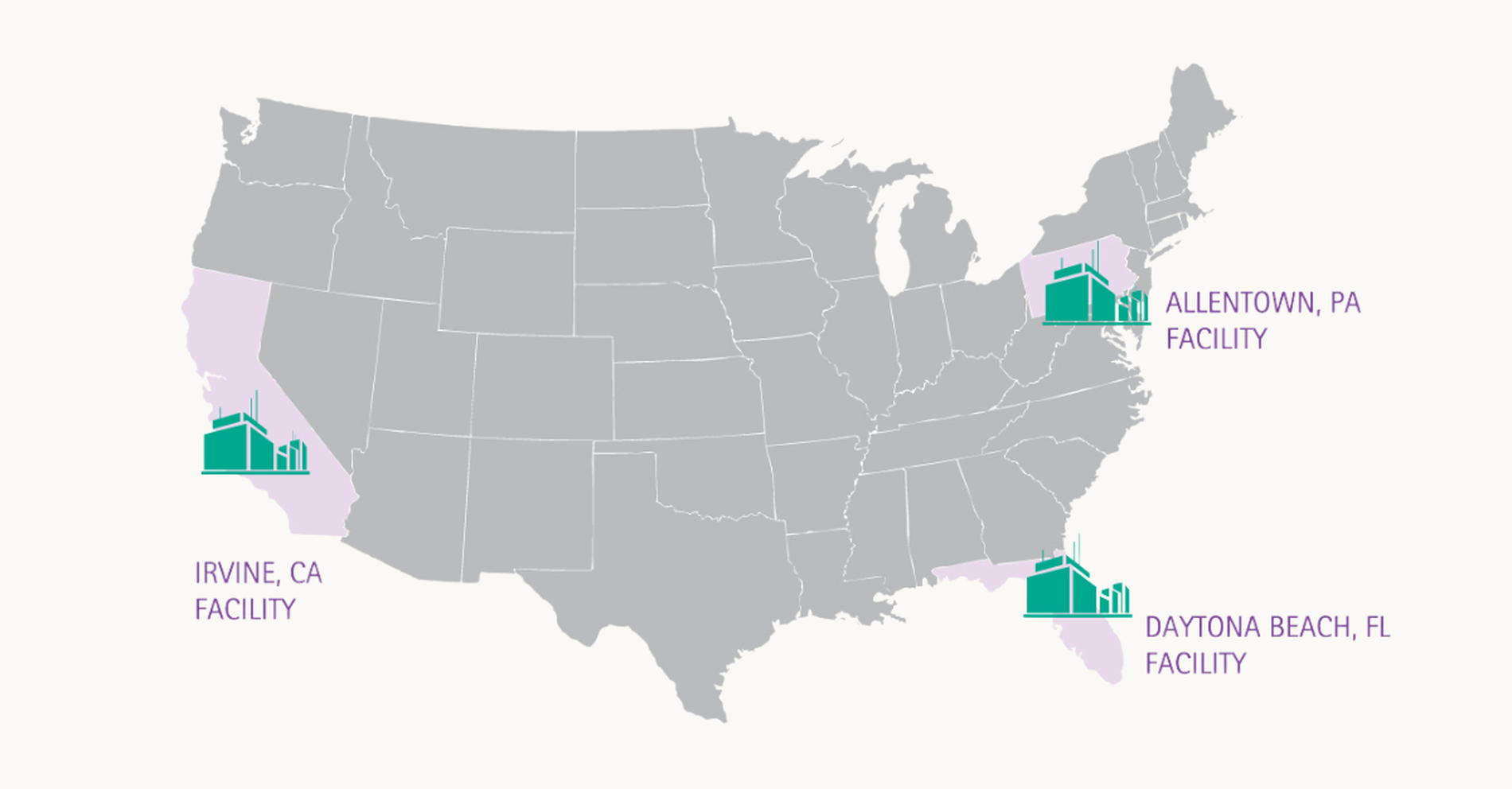
Our supply chain commitment stretches from coast to coast
We provide a redundant, more reliable supply of vital products for healthcare providers and the patients they serve.
Learn moreReducing our environmental impact
Our commitment to sustainability is evident in everything we do. As a company, we believe that reducing our environmental footprint and taking responsibility for current and future generations is necessary, not only to protect people and our planet but also to enable our continued growth.
Learn more